Biologics Research Unit
Overview

Application of Biopharmaceutical Research Findings to the Development of New Modalities, Complex Peptide Drugs and Nucleic Acid Medicines, and Nanoparticles
Biologics is a pharmaceuticals produced using animal cells, etc., and is characterized by its complicated structure compared to small molecules. In recent years, Biopharmaceuticals represented by antibody drugs has been in the limelight.
Recently, the main research subject is shifting to "next-generation antibody drugs," "gene therapy drugs," and "cell drugs" for cell therapy, which are developed under the same ICH guidelines. And this department is conducting research focusing on these manufacturing process. Furthermore, we are also approaching peptide drugs and nanotechnology + nucleic acid drugs to which research methods similar to biopharmaceutical production can be applied.
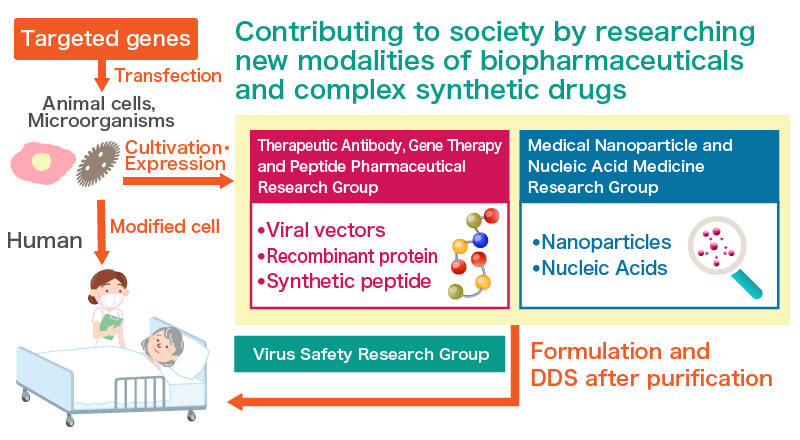
Research Groups
Therapeutic Antibody, Gene Therapy and Peptide Pharmaceutical Research Group
This research group is responsible for the development of a manufacturing process for engineered proteins (which are useful biopharmaceuticals using CHO cells), analysis of said process, research on quality analysis techniques, and study into the continuation of culturing and purification processes for the latest antibody drugs. By making the most of an advantage of microorganisms—rapid cell growth—this research group is also developing strains for the generation and production of biopharmaceuticals using the methylotrophic yeast Pichia pastoris.
Other research topics include production and analysis of vectors for gene Therapy, whose processes can be designed in a similar way to that of protein drug cultures and purification processes, with a focus on nucleic acid analysis methods for vectors (active substances). Subjects for study by the group are adeno associated viruses (DNA viruses) and lentiviruses (retroviruses). The group is also conducting investigative research on production and analysis of peptide pharmaceuticals.
Members
YAMAJI Hideki (Professor), UCHIDA Kazuhisa(Professor), KONDO Akihiko (Professor), ISHII Jun (Associate Professor), ITO Yoichiro (Associate Professor), and KATAYAMA Kenta (Assistant Professor)
Virus Safety Research Group
In accordance with the International Conference on Harmonization (ICH) Guidelines Q5A, laboratories of biosafety level (BSL) 2, where mouse leukemia retroviruses and mouse minute viruses may be handled, have been constructed to conduct research virus safety evaluation methods, such as biopharmaceutical virus clearance testing.
Furthermore, in the field of biologics such as biopharmaceuticals and cell therapies, this group uses next-generation sequencing methods and bioinformatics to establish novel virus quality evaluation techniques. By sharing research outcomes at international consortiums, this group is working to provide input on the next version of biosafety guidelines.
Members
UCHIDA Kazuhisa (Professor), and YUSA Keisuke (Professor)
Medical Nanoparticle and Nucleic Acid Medicine Research Group
This research group is attempting to develop a drug delivery system (DDS) that uses nanoparticles to efficiently transport the maximal amounts of anticancer drugs to tumors in order to enhance therapeutic effects and mitigate adverse drug reactions. Other than anticancer drugs, “nucleic acid medicines,” which contain nucleic acids that control gene expressions in nanoparticles, are being developed. This group also conducts research and investigations regarding the synthetic process of nucleic acid medicines inside nanoparticles, i.e., cost reduction by mass production and maintenance of superior quality. Also under development is imaging technology for cancer diagnosis using metal composite nanoparticles.
Member
OGINO Chiaki (Professor)
Please see entries under the Chemical and Process Research Unit of the Members page for Professor OGINO Chiaki’s profile.
